Penta Medical
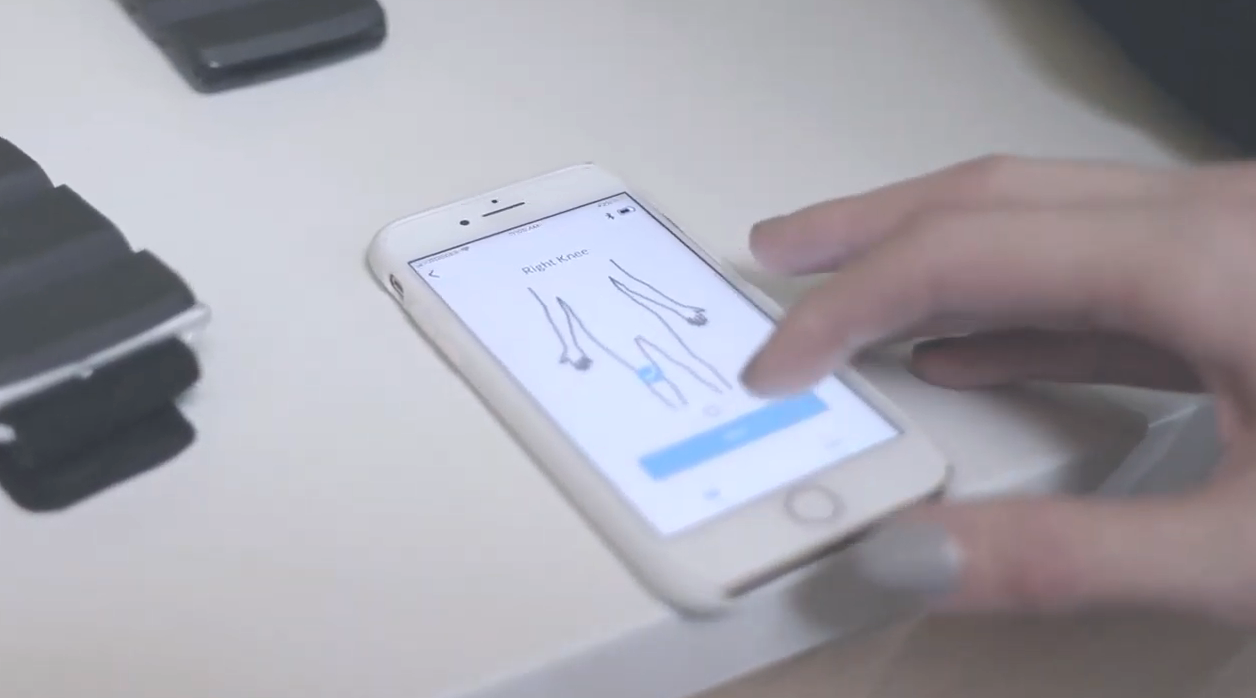
Penta Medical produces and sells a mobile app & portable device that treats and monitors soft tissue injuries. The product uses Low-Level Laser Therapy (LLLT), which is a treatment commonly used in physical therapy clinics. Penta is the first at-home system that meets the same standard as the devices used in clinics. The device is controlled by the mobile app, which allows it to use the information patients provide about their injuries to adjust their treatment level. Usage data is then sent back to the care provider the patient is linked to, and displayed in-app so both parties can follow the injury progress.
The idea for the product was developed while I was studying biotechnology at the University of Waterloo, based on personal experience with fractured injury recovery monitoring in the healthcare system. I hired small engineering team to build out the device based on design parameters from focus groups and regulatory requirements. We worked with a consultant to help us navigate the regulatory pathway, and found which design rules to follow to allow us to fall into one of the lowest risk classes, which is critical as we wanted the device to be a consumer, at-home device.
Manufacturing and regulatory can severely impact timelines of any product, especially medical devices. We were strategic about our plan in several ways:
Our product was designed from the beginning to fit into the 2nd lowest risk class in the FDA, allowing us to skip past clinical trials. The treatment technology we were using (LLLT) is already proven and approved.
Instead of attempting to manufacture our own devices, we partnered with a medical device contract manufacturer. This allowed us to skip past getting our own ISO certificates and medical manufacturing licences, produce small batches fairly economically, and achieve quick iteration turnaround times.
Our device design is small and modular, so one device could be used anywhere on the body, which meant we only needed one hardware product for many use-cases.
Once Penta was in-market, the mobile app quickly became the most valuable part of our product. Our early customers wanted the app to be able to track the data the patient was inputting to use the app (e.g. how their pain level was that day), and to be able to see how frequently patients were using the device to help them understand at-home compliance. At-home compliance is impossible to measure accurately when asking patients to self-report; using our device, we could automate the care providers’ ability to see treatment frequency. This helped care providers understand if an injury progression was due to patient error or complications with the injury itself, which affects treatment plans.
Sports Medicine became our preferred focus group due to their appetite for leading-edge products and significant financial interest in recovery times. We partnered with Michigan State University Athletics for product feedback, who became our first customer. They had connections with professional sports teams in the US, and the product took off within MLB teams. They had the perfect use case of high monetary value associated with their injuries, which made them an ideal fit for a product like ours. Through the MLB teams, we proceeded to sell to several high end physical therapy clinics in the US where the product is available to consumers.
